
Ethics of Research Involving Human Participants Policy
– May 28, 2024 of 5
ETHICS OF RESEARCH INVOLVING HUMAN
PARTICIPANTS POLICY
Policy Type:
Management
Initially
Approved:
May 10, 2012
Policy
Sponsor:
Provost and Vice-
President,
Academic
Last
Revised:
May 28, 2024
Primary
Contact:
AVP, Research,
Scholarship and
Community
Engagement
Review
Scheduled:
May 28, 2029
Approver:
Board of Governors
A.
OVERVIEW
Mount Royal University (the University) is committed to advancing human welfare, knowledge and
understanding through research. The University recognizes that Human Participants are
fundamental to this process and that all research involving Human Participants must be conducted
with the highest standards of ethical conduct that respect human dignity and well-being.
The University endorses and upholds the current official Tri-Council Policy Statement (TCPS 2):
Ethical Conduct for Research Involving Humans, and maintains a Human Research Ethics Board
(HREB) to ensure that all research investigations involving human participants are carried out in
compliance with the TCPS 2
and the University’s policies.
B.
SCOPE
This Policy applies to all Employees, Students and individuals affiliated with the University (e.g.,
research associates, emeriti, adjuncts, visiting scholars, etc. hereinafter referred to as Members)
participating in research that involves human participants at or under the auspices of the University:
• where such research is conducted by members or associated members of the University
acting in their university capacity;
• where such research is conducted at the University, its affiliated sites, or through its
systems of distributed learning;
• where such research is administered by the University; or
• where ethics clearance by the University is required for research pursuant to an affiliation
agreement with other agencies.
C.
PURPOSE
This Policy is intended to:
• create a research environment in which human research participants are protected;
• ensure responsibilities are discharged according to the relevant ethical standards, by
promoting awareness of research ethics amongst faculty, staff and students, establishing
an independent research ethics review process;

Ethics of Research Involving Human Participants Policy
– May 28, 2024 of 5
• establish mechanisms for the protection of human participants in research.
D.
GUIDING PRINCIPLES
Over and above the legal obligations to which all researchers and the University are bound to
adhere, the central imperative of Research Involving Human Participants is the respect for human
dignity.
The University adopts the Tri-Council Core Ethical Principles (see Definitions) as principles that
guide both the conduct of all Research Involving Human Participants and HREB when reviewing
the ethical acceptability of such research under its auspices.
E.
POLICY STATEMENT
1. RESEARCH INVOLVING HUMAN SUBJECTS
1.1. The University will regulate the conduct of all Research Involving Human Participants in
accordance with the most current version of the Tri-Council Policy Statement: Ethical
Conduct for Research Involving Humans and, where applicable to specific research, other
relevant national and international standards.
1.2. .
No research to which this Policy applies may be undertaken, nor may University services
or facilities be used, nor may funds for such purposes be released, nor financial accounts
opened unless the research has received formal ethical clearance from HREB before the
proposed research begins and the research has received a Letter of Clearance.
1.3. Any material changes in research to which this policy applies, as proposed, must have
clearance of the University’s HREB.
2. MANDATE AND AUTHORITY OF THE UNIVERSITY HUMAN RESEARCH ETHICS BOARD
2.1. The HREB is established by the Board of Governors of Mount Royal University and
empowered to ensure that all research conducted under the auspices of the University is
designed and conducted in such a manner that it protects the rights, welfare and privacy
of research participants. HREB has the authority to approve, reject, propose modifications
to, or terminate any proposed or ongoing Research Involving Human Participants that is
conducted within, or by members of, the University as outlined in this Policy and TCPS 2.
2.2. The University shall ensure that those who conduct such research understand their
responsibilities for the ethical conduct of their research, and receive appropriate training,
as indicated by the HREB, in the skills necessary for such conduct. This includes not only
awareness of, but understanding of the relevant policies and professional standards.
3. RECOGNITION OF DECISION OF EXTERNAL RESEARCH ETHICS BOARD
3.1. Where an approval for research involving humans is required from a research ethics board
pursuant to this Policy, and where Ethics Approval is also required from a research ethics
board of one or more other institutions pursuant to the human research ethics policy of
that other institution(s) - sometimes referred to as multi-jurisdictional research reviews -
HREB may accept the review of the research ethics board of one of those other institutions
if permitted by, and in accordance with the requirements of, the TCPS. For more than

Ethics of Research Involving Human Participants Policy
– May 28, 2024 of 5
minimal risk research, REB authorization should be based on an official agreement (TCPS
2, Article 8.1)
F.
DEFINITIONS
(1)
Ethics Approval:
Research ethics approval granted by HREB in accordance with
this Policy.
(2)
Human Participants:
An individual whose data, biological materials, or responses to
interventions, stimuli, or questions by a researcher are relevant
to answering the research question(s) (from TCPS 2 Glossary).
(3)
HREB:
The human research ethics board authorized by the University.
(4)
Letter of Clearance
Means the research ethics approval granted by a REB in
accordance with this Policy.
(5)
Member
Means any individual who teaches, studies, conducts research;
all Employees, contractors, volunteers, and visitors to the
University; and any other individual acting on behalf of the
University.
(6)
Minimal Risk:
Minimal Risk research is defined as research in which the
probability and magnitude of possible harms implied by
participation in the research is no greater than those
encountered by the participant in those aspects of their everyday
life that relate to the research (from TSPS 2 Glossary).
(7)
Policy:
means the Ethics of Research Involving Human Participants
Policy.
(8)
Research:
research is an undertaking intended to extend knowledge
through a disciplined inquiry and/or systematic investigation
(from TCPS 2 Glossary).
Research also includes activities related to one or more of
scholarly or artistic work which occurs through discovery,
integration, teaching and learning, or application of knowledge
and is usually disseminated through peer-reviewed processes.
(9)
Tri-Council Policy
Statement 2 (TCPS
2):
The Tri-Council Policy Statement: Ethical Conduct for Research
Involving Humans (TCPS or the Policy) is a joint policy of
Canada’s three federal research agencies – the Canadian
Institutes of Health Research (CIHR), the Natural Sciences and
Engineering Research Council of Canada (NSERC), and the
Social Sciences and Humanities Research Council of Canada
(SSHRC), or “the Agencies.” The current edition, TCPS 2,
introduces the second set of substantive changes to the Policy
(from TCPS 2 Introduction).
.
(10) Tri-Council Core
Ethical Principles:
Respect for Persons: A core principle of this Policy that
recognizes the intrinsic value of human beings and the respect
and consideration that they are due. It incorporates the dual
moral obligations to respect autonomy and to protect those with

Ethics of Research Involving Human Participants Policy
– May 28, 2024 of 5
developing, impaired, or diminished autonomy (from TCPS 2
Glossary)..
Concern for Welfare: A core principle of TCPS 2 that requires
researchers and research ethics boards to aim to protect the
welfare of participants, and, in some circumstances, to promote
that welfare in view of any foreseeable risks associated with the
research (from TCPS 2 Glossary).
Justice: A core principle of TCPS 2 that refers to the obligation
to treat people fairly and equitably. Fairness entails treating all
people with equal respect and concern. Equity requires
distributing the benefits and burdens of research participation in
such a way that no segment of the population is unduly burdened
by the harms of research or denied the benefits of the knowledge
generated from it (from TCPS 2 Glossary).
(11) University:
means Mount Royal University
G.
RELATED POLICIES
•
Responsible Conduct in Research Policy
•
Enterprise Risk Management Policy
H.
RELATED LEGISLATION
•
Alberta Freedom of Information and Protection of Privacy Act
I.
RELATED DOCUMENTS
•
Procedures for Responsible Conduct in Research
•
Procedures for Conflict of Interest in Research
•
Procedures for the Collection, Storage and Authenticity of Research Data
•
Application for Ethics Review
•
Completion Report
•
Letter of Clearance
•
Office of Research Services Tracking and Signature Form
•
Ethics of Research Involving Human Participants Procedures
– two documents
•
Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans
•
University of Calgary’s Research Ethics Appeal Board Policy
Ethics of Research Involving Human Participants Policy
– May 28, 2024 of 5
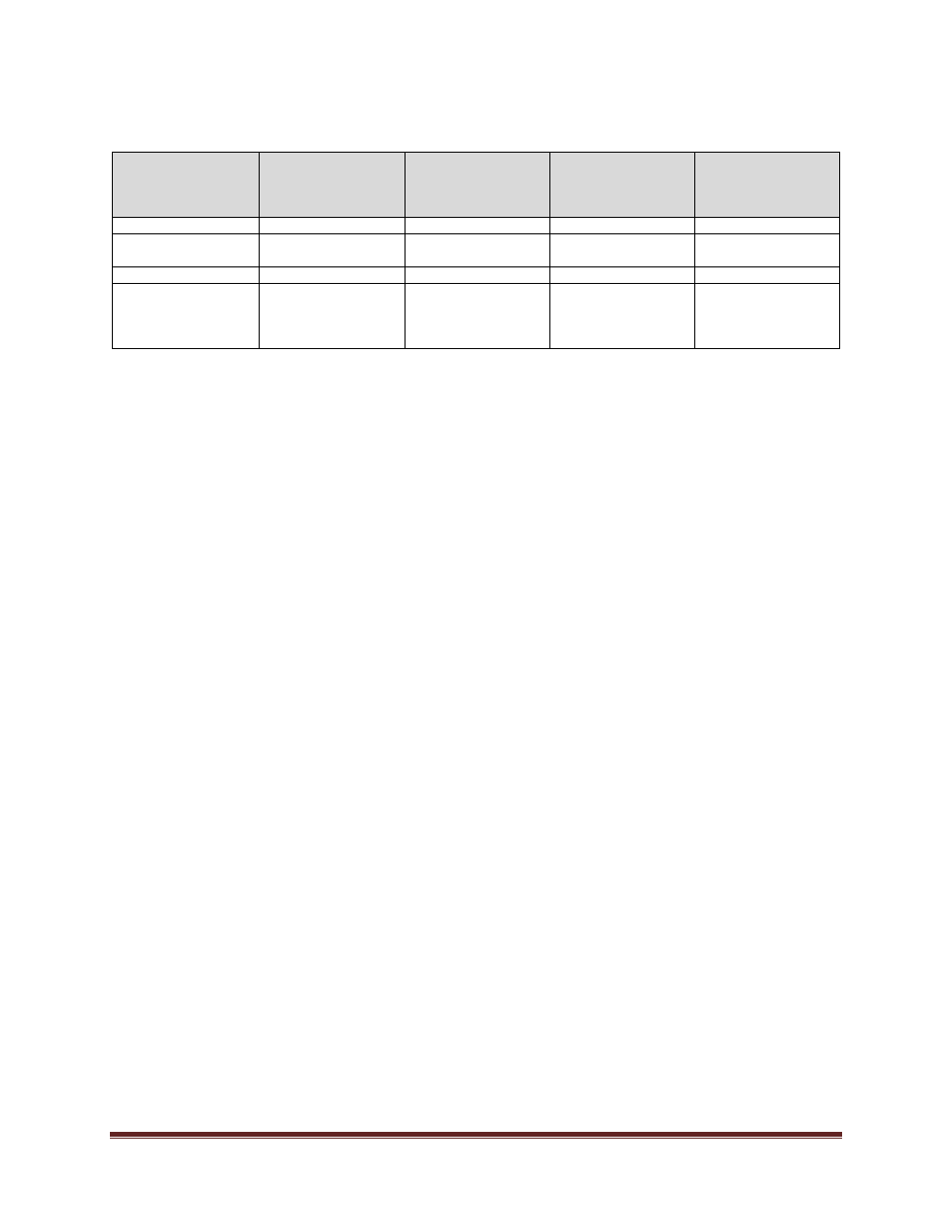
J.
REVISION HISTORY
Date
(mm/dd/yyyy)
Description of
Change
Sections
Person who
Entered Revision
(Position Title)
Person who
Authorized
Revision
(Position Title)
05/100/2012
NEW
08/31/2017
Editorial
– formatting
Director, University
Secretariat
01/22/2020
Editorial
Template Update
Policy Specialist
University Secretary
05/28/2024
Comprehensive
revisions
All
AVP Research
Scholarship and
Community
Engagement
Board of Governors




