Human Research Ethics Board (HREB)
The MRU Human Research Ethics Board (HREB) is responsible for reviewing the ethical acceptability of all research involving humans conducted within its jurisdiction or under its auspices, that is, by MRU faculty, staff, or students, regardless of where the research is conducted, in accordance with TCPS2. HREB's mandate is to ensure that research conducted under the auspice of Mount Royal University is designed and carried out in such a manner that protects the rights, welfare, and privacy of research participants in compliance with the Tri-Council Policy Statement (TCPS2).
All faculty members, staff, and students at the University must have HREB ethics approval to conduct research involving human participants (or their data) before the research commences. This requirement applies to all individuals conducting research in connection with the University,
- anywhere on-campus or off, regardless of location
- in person or by mail, telephone or online
- regardless of the type of research (eg. experimental, correlational, qualitative, descriptive, pilot project, or a fully formed study)
- whether the results are intended for publication or not
External Applications
All MRU researchers must have HREB ethics approval before engaging in research as part of a team at external institutions. Any research carried out at MRU, even while approved at an external post-secondary institution in Canada, must be reviewed by HREB before the research commences. Use the external application process if you:
- are an MRU researcher working as part of research teams at other institutions
- wish to conduct research with Mount Royal University participants and your study has been approved at an external post-secondary institution in Canada.
The Human Research Ethics Board (HREB) is responsible for ensuring that research applications meet the Tri-Council Policy Statement (TCPS2) involving participants, their rights and well-being. Learn more about HREB.
Members
| Name | Department | Phone | |
|---|---|---|---|
| Lynne Lafave (HREB Chair) | Health and Physical Education | 403.440.5967 | llafave@mtroyal.ca |
|
Harris Rubin (HREB Vice Chair and SHREC Chair) |
General Management and Human Resources | 403.440.6658 | hrubin@mtroyal.ca |
| Hapreet Aulakh | Economics, Justice and Policy Studies | 403.440.5907 | haulakh@mtroyal.ca |
| Dan Devoe | Psychology | ddevoe@mtroyal.ca | |
| AnneMarie Dorland | Marketing | 403.440.6960 | adorland@mtroyal.ca |
| Aliyah Dosani | Nursing and Midwifery | 403.440.8631 | adosani@mtroyal.ca |
| Mariam Elhussein | Mathematics and Computing | 403.440-6918 | melhussein1@mtroyal.ca |
| Mohamed El-Hussein | Nursing and Midwifery | 403.440.8633 | melhussein@mtroyal.ca |
| Jared Fletcher | Health and Physical Education | 403.440.5514 | jfletcher@mtroyal.ca |
| Cass Foursha-Stevenson | Psychology | 403.440.8829 | cfourshastevenson@mtroyal.ca |
| Lauren Goldade | Community Member | ||
| Naomi Grant | Psychology | 403.440.8837 | ngrant@mtroyal.ca |
| Sarah Hamilton | Education | 403.440.5691 | shamilton1@mtroya.ca |
| Giuliana Harvey | Nursing and Midwifery | 403.440.8622 | gharvey@mtroyal.ca |
| Emma Kamanja | Community Member | ||
| Trevor King | Health and Physical Education | 403.440.6227 | tking@mtroyal.ca |
| Gabrielle Weasel Head | Humanities | 403.440.6536 | glindstrom@mtroyal.ca |
| Matthew McLarnon | General Management and Human Resources | 403.440.6991 | mmclarnon@mtroyal.ca |
| David Ohreen | Humanities | 403.440.8645 | dohreen@mtroyal.ca |
| Elaine Ori | Health and Physical Education | 403.440.6508 | eori@mtroyal.ca |
| Ines Sametband | Psychology | 403.440.5962 | isametband@mtroyal.ca |
| Elisa Twoyoungmen | Community Member | ||
| Jim Silovs | International Business, Supply Chain Management & Aviation | 403.440.8782 | jsilovs@mtroyal.ca |
| Priscilla Wamucii | Research Compliance Officer | 403.440.8470 | hreb@mtroyal.ca |
Ad Hoc Advisors
| Name | Department | Phone | |
|---|---|---|---|
| Stacey Page, Chair, CHREB | University of Calgary Conjoint Health Research Ethics Board (CHREB) | 403.220.2763 | sapage@ucalgary.ca |
| Brian Jackson | Library (Data Management Specialist) | 403.440.5032 | bjackson@mtroyal.ca |
Application Process
Ethics application forms can be accessed through the MRU ROMEO portal. Instructions on how to use the portal can be found in the ROMEO user guide page.
Note: Applications that are incomplete, or those where significant methods concerns are noted, will be returned to applicants with a request to revise and re-submit prior to ethics reviewed by the HREB.
The following links provide information on submitting and managing ethics applications at Mount Royal University.
- Log in to ROMEO.
- Click here to create a new ROMEO account. If you already have a ROMEO account, click here to log in. For further instructions, please see the ROMEO user guide.
- Link your HREB application to an Activity Form Number- Choose the most appropriate option
o Create and use a new Research Activity Form Number - if your study is
-
-
-
- funded through an external grant (e.g., CIHR, SSHRC, etc.).
- not funded
-
-
o Use your existing ROMEO Research Award Number – if your study is associated with an existing ROMEO Award Form
- Complete the HREB application in ROMEO and submit.
- Use the Attachment Tab to upload consent and debriefing forms, ethics training certificate and other materials associated with your application.
- You will not be receiving a confirmation number after submitting the HREB application.
- An acknowledgement email will be sent to you from the Compliance Officer stating that the application has been received and is undergoing initial screening.
- The Initial screening process will take approximately 3-10 days depending on the volume of the received applications.
- If the received application is incomplete, you will receive an email from the Compliance Officer asking for clarification (e.g., please send a consent form with your application).
- Once the screening process is complete and the Compliance Officer's concern have been addressed, the Compliance Officer will send the application out for review. It may delegated to one or two people, or a full board.
- The default is a blind review process, but it is possible that reviewers will identify themselves.
Delegated Review
- A Reviewer Report will be completed by HREB reviewer(s) approximately four-six weeks after an application has been submitted and copied into ROMEO.
- The Chair will assess the completed Reviewer Report. If any additional concerns are noted, they will be discussed with the reviewer.
- An email will be sent to the researcher stating that the review is available in ROMEO and will include instructions to review the Reviewer Report and make any necessary changes to the application in ROMEO:
- The changes will be visible to both the researcher and his/her reviewer(s) using the Log tab in ROMEO.
- When the revisions are complete, click the Submit button to re-submit your application.
- The Compliance Officer will be notified and the revised application will be sent to reviewer(s).
- The HREB application will be reviewed again. If any requested changes have been missed, the reviewer(s) will indicate this at the top of the review in ROMEO.
- When the reviewer is satisfied that the application meets the requirements of TCPS-2, the reviewer will notify the Compliance Officer and HREB Chair. The Chair will review and approve the application.
- The researcher will receive an email with the clearance letter attached generated by ROMEO. The letter will also be available on the researcher's Attachment tab. The project may be started once the letter has been received.
- Progress and completion form templates are available in ROMEO.
- Researchers may log in to the Research Portal at any time to check the status of their application and to determine when the progress on study completion reports are due.
- Note: Any ethical concerns that arise during the project should be reported directly to HREB (Compliance Officer and Chair).
- Modification requests will be submitted via ROMEO. These will be reviewed by the Compliance Officer and/or the Chair (estimated review time is one week).
Full Board Review
Full Board Review takes approximately two months to get an initial review response. Here are some details about a Full Board Review process:
- Applications are judged on the level of risk involved. In general, Full Board Reviews are required for high risk and ethically complex studies, or where diverse opinions across researchers are likely. This decision is admittedly subjective. Whether or not an application goes to Full Board or Delegated review, the decision rests with the REB Chair. Possible Full Board Review applications may also be flagged by an REB member during a delegated review or the Research Compliance Officer during the screening process.
- Board meetings are generally held the first Friday each month. Please check the website for the upcoming HREB meeting dates. An application requiring Full Board Review must be submitted at least two weeks before the monthly HREB meeting. NOTE: Meeting the Full board submission deadline does not guarantee review at the next HREB meeting.
Note: Meeting the Full Board submission deadline does not guarantee review at the next HREB meeting. Full Board Review of the application will be dependent on the soundness of the application after screening, and/or any significant constraints on the HREB meeting agenda.
The deadlines for applications that require HREB review for 2024/2025 are as follows:
- August 19, 2024
- September 18, 2024
- October 9, 2024
- November 13, 2024
- December 11, 2024
- January 15, 2025
- February 12, 2025
- March 12, 2025
- April 9, 2025
- May 9, 2025
Notes to Remember
- ROMEO has a 10MB limit. Large PDF files (supporting materials) might need to be split into several smaller documents before they are uploaded.
- Course Based Applications are reviewed by the Student Human Research Ethics Committee and must be submitted on ROMEO
- Log in to ROMEO.
- Under the Role: Principal Investigator tab, click Applications: Post-Review.
- A list of all your reviewed projects will show up on the screen. Select the file you want to modify and click Events.
- Under Create New Event, click on Ethics Modification.
- Fill out required information for all the tabs. Notes to remember:
- Attachments tab
- Upload all revised documents (PDF versions) here. This includes submission of the revised *application form* with version date.
- Use 'track changes' or 'colour highlights' to identify what you are removing/replacing in the *approved application form* (use the 'export to word' tab to access the word version of the approved application form.
- Attach all supporting documents (e.g., survey questions, recruitment script, consent forms, etc.)
- In addition to attaching tracked copies of your application form and other documents, attach their corresponding clean/final (no mark-up/highlighting showing) versions where the proposed changes have been implemented
- Logs tab
- This can be used to follow the application through the approval process
- No information is needed on this tab
- Dates will be completed automatically throughout the modification process
- Attachments tab
- When all required information have been filled, click Submit.
- A pop-up Comment box will appear. You must type something in the box to proceed.
- You will receive an email confirmation stating your application has been successfully submitted. Your application will be forwarded to the Compliance Officer for further processing. You can keep track of your approval process through the Logs tab within your application.
- If clarifications on your modifications are required, your application may be returned to you again. If no further clarification are required, you will receive a ROMEO notification on the Logs tab granting approval of the requested modification and an email with your clearance letter enclosed.
Progress reports are annual progress reports required by the TCPS2 ethics guidelines during your studies and are needed for annual study extensions, while completion reports are required after the termination of the study.
- Log in to ROMEO.
- Under the Role: Principal Investigator tab, click Applications: Post-Review.
- A list of all your reviewed projects will show up on the screen. Select the file you want to modify and click Events.
- Under Create New Event,
- For progress reports: Click Ethics Progress Report
- For completion reports: Click Ethics Completion Report
- Fill out all required information. Notes to remember:
- Attachments tab: Upload all additional documents (PDF versions) that needs to accompany your progress report
- Logs tab: Use this to follow the application through the approval process. No information is needed on this tab. Dates will be completed automatically through the modification process
- Errors tab: Lists all the fields that need to be completed before the progress can be successfully submitted
- When all required information have been filled, click Submit.
- A pop-up Comment box will appear. You must type something in the box to proceed
- You will receive an email confirmation stating your application has been successfully submitted. Your application will be forwarded to the Compliance Officer for further processing. You can keep track of your approval process through the Logs tab with your application.
- If clarifications are required, your application may be returned back to you again. If no further clarifications are required,
- For progress reports: You will receive your one year extension notification clearance through a ROMEO notification on the Logs tab granting extension approved for your study or through email.
- For completion reports: You will receive your notification through a ROMEO notification on the Logs tab or through email.
External Application ProcessThe Human Research Ethics External Application Form is to be used in three instances:
- MRU researchers working as part of a multi-jurisdictional research team with minimal risk research studies that have been approved at an external TCPS 2 accredited institution and conducted entirely in Canada, can submit through the external research application process;
- MRU researchers working as part of a multi-jurisdictional research team with minimal risk research studies that have been approved at an external TCPS 2 accredited institution and conducted in whole or in part outside of Canada, can submit through the external research application process;
- Non-MRU researchers, who have been granted ethics clearance to recruit members of the MRU community from an external TCPS 2 accredited institution, must submit an ethics application to HREB through the external research application process.
Note:
- The research protocol must be unchanged from the externally reviewed application
- MRU researchers who are part of a research team where the research ethics application has received ethical clearance internationally or from a non-TCPS 2 accredited institution will submit their application through the full HREB application process.
External Application Requirements
- Approval letters, approved application forms, and all supporting documents (recruitment advertisements, consent forms, questionnaires, interview guides, debriefing forms, etc) from the approving TCPS 2 accredited institution must be submitted as part of the external application;
- MRU members are required to submit a Research Activity Form (RAF) number as part of this application (both funded and unfunded). The RAF form link can be found in Romeo.
External Application Steps
- Members of the MRU community - use your myMRU login and password via this link.
- Non-MRU researchers will now access ROMEO via this link.
If you require assistance with this process, please contact us at hreb@mtroyal.ca
Multi-Jurisdictional Research
Sometimes researchers work in teams across institutions either entirely within Canada, or in Canada and other countries. Multi-jurisdictional research ethics review extends to research that is associated with students, faculty, and staff under the auspices of Mount Royal University. MRU is responsible for the ethical acceptability and ethical conduct of research undertaken within its jurisdiction or under its auspices irrespective of where the research is conducted. This encompasses the use of MRU resources (e.g., recruiting through MRU faculties, departments, associations etc.), use of funds administered through MRU, and research conducted using MRU’s physical resources (e.g., space).
Multi-Jurisdictional Research Conducted within Canada
TCPS 2 (2022) provides the opportunity to adopt alternative review models for research involving multi-jurisdictional research (Article 8.1) for minimal risk research. HREB has implemented an alternative review model in accordance with this policy. In order to determine the approach an MRU researcher should take; it is important to decide which institution is the lead institution for the purposes of ethics review. The principal investigator (PI) is defined as the researcher who is responsible for the ethical conduct of the research, and for the actions of any member of the research team.
There are two approaches to multi-jurisdictional research ethics review:
1) When the PI is based at MRU – the ethics application is submitted through the full HREB application process, includes the list of all external research team members, describes their roles in the research project, and describes how research data will be managed within the team. Ethics review at the research collaborator(s) institution will follow their local REB processes.
2) When the PI is based at an external TCPS 2 accredited institution - the ethics application is submitted to the external institution first, lists MRU members as potential partners, and describes how data is to be managed amongst the team. Once cleared at the external PI’s TCPS 2 accredited institution – the MRU researcher submits an external application for streamlined review.
Note: for more than minimal risk research, official agreements between institutions reviewing the multi-jurisdictional research is required. In the absence of an official agreement, applications follow the full HREB application process. This will be assessed on a case-by-case basis; the first order of contact should be with the HREB Research Compliance Officer.
Multi-Jurisdictional Research Conducted Internationally
MRU researchers may undertake research at numerous sites within Canada and in countries around the world. Such research may be carried out with or without any collaboration with host institutions and local researchers. In exercising its responsibilities for the initial and continuing ethics review of research conducted under its auspices, HREB must satisfy itself that the requirements of TCPS 2 (2022) are met, both at MRU, and within the other country or research site. HREB is required to take appropriate steps to ensure researchers are responsive to ethically relevant aspects of the research context in accordance with TCPS Article 8.3.
There are two approaches for the review of minimal risk multi-jurisdictional research conducted internationally:
1) When research is conducted by MRU researchers and performed in whole or in part outside of Canada – the ethics application is submitted through the full HREB application process and the REB or other responsible review body or bodies, if any, at the research site. (Follow this process if the application has received ethical clearance internationally).
2) When research that will be conducted by MRU researchers and performed in whole or in part outside of Canada is approved at an external TCPS 2 accredited institution under a multi-jurisdictional review model – the MRU researcher submits an external application for streamlined review.
Note: for more than minimal risk research, official agreements between institutions reviewing the multi-jurisdictional research is required. In the absence of an official agreement, applications follow the full HREB application process. This will be assessed on a case-by-case basis; the first order of contact should be with the HREB Research Compliance Officer.
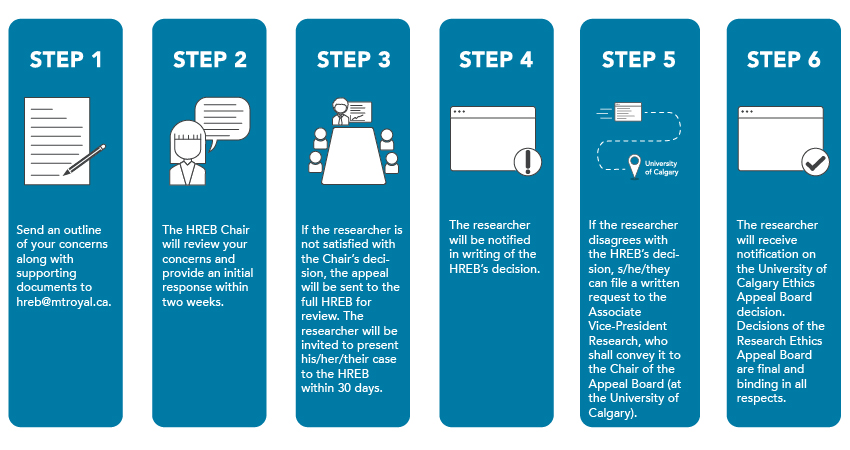
Appeal Process
We recognize that disagreements between researchers and the HREB over a decision can arise from time to time. If a disagreement cannot be resolved between the researcher and reviewer, the following process should be followed:
Ethics Knowledge and Training
MRU researchers are responsible to be knowledgeable of and follow the ethical conduct for research involving humans as outlined in the Tri-Council Policy Statement as outlined in TCPS 2 (2022).
TCPS 2 (2022)
The Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2 - 2022) provides ethical guidance for all research involving human participants, including their data and/or biological materials. This guidance applies to research conducted under the auspices of institutions eligible for funding from the federal Agencies: CIHR, NSERC, and SSHRC.
TCPS 2: CORE-2022 (Course on Research Ethics)
The CORE-2022 (Course on Research Ethics) is an online tutorial providing an introduction to the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2). It covers essential TCPS 2 ethical guidance applicable to all research involving human participants, regardless of discipline or methodology.
Important Update: Effective January 1, 2024, submissions to the Human Research Ethics Board (HREB) must include the CORE-2022 Certificate of Completion as part of the application package.
- Click here to access the TCPS 2 (2022) (CORE Tutorial) Online Training Modules
Specialized Training
Custom sessions on specific topics from TCPS2 for groups are available by request post CORE tutorial training.
If you have any questions or require more information on ethics and compliance, please contact the Research Compliance Officer at 403.440.8470 or hreb@mtroyal.ca

